CRE ACTIONS
 In December 2014, the Critical Care Research Group (CCRG) was awarded a prestigious $2.5 million National Health and Medical Research Council, Centre of Research Excellence (CRE) in Advanced Cardio-respiratory Therapies Improving OrgaN Support (ACTIONS). CRE ACTIONS is a unique, global level collaboration of clinical, engineering and scientific experts in Ventricular Assist Devices (VAD) and Extra Corporeal Membrane Oxygenation (ECMO) who together with patients supported by these therapies, is addressing the range of key challenges associated with the clinical implementation of these technologies, to accelerate the translation of knowledge from the laboratory to the bedside.
In December 2014, the Critical Care Research Group (CCRG) was awarded a prestigious $2.5 million National Health and Medical Research Council, Centre of Research Excellence (CRE) in Advanced Cardio-respiratory Therapies Improving OrgaN Support (ACTIONS). CRE ACTIONS is a unique, global level collaboration of clinical, engineering and scientific experts in Ventricular Assist Devices (VAD) and Extra Corporeal Membrane Oxygenation (ECMO) who together with patients supported by these therapies, is addressing the range of key challenges associated with the clinical implementation of these technologies, to accelerate the translation of knowledge from the laboratory to the bedside.
About
The Critical Care Research Group (CCRG) at The Prince Charles Hospital, Brisbane, Australia is the largest multidisciplinary critical care research team in Australasia.
Cardiovascular disease is responsible for 11 per cent of health expenditure in Australia (2008/9); the burden of disease it imposes in terms of disability and premature death is second only to cancer. It is responsible for more deaths (30 per cent of all deaths) in Australia than any other disease group (Heart Foundation). The gold standard treatment for end-stage heart and lung failure is transplant however falling organ donation numbers gives rise to the need for artificial hearts and lungs to support our most critically ill patients in the management of acute and chronic end stage cardio-respiratory disease and as a bridge to transplantation.
Artificial hearts and lungs are increasingly used to support our most critically ill patients. The rapid and continuous expansion of these Mechanical Assist Devices (MADs), such as Extra Corporeal Membrane Oxygenation (ECMO) and Ventricular Assist Devices (VADs), in the management of acute and chronic end stage cardio-respiratory disease highlights the potential benefit to patients from research in this field. The barriers of complexity, cost and risk combine to inhibit the more widespread use of these devices.
As the ageing and ailing community of the 21st century increasingly depends on mechanical assistance in many forms, a greater understanding of patient-machine interaction is essential if this technology is to deliver its full potential. In December 2014, the CCRG was awarded a prestigious $2.5 million National Health and Medical Research Council, Centre of Research Excellence (CRE) in Advanced Cardio-respiratory Therapies Improving OrgaN Support (ACTIONS). CRE ACTIONS is a unique, global level collaboration of clinical, engineering and scientific experts in Ventricular Assist Devices (VAD) and Extra Corporeal Membrane Oxygenation (ECMO) who together with patients supported by these therapies, is addressing the range of key challenges associated with the clinical implementation of these technologies, to accelerate the translation of knowledge from the laboratory to the bedside.
CRE ACTIONS is the first mechanical cardio-respiratory support CRE in Australia based in a hospital and is researching device-related complications, improving device components, developing clinical practice guidelines, training clinical and engineering researchers and exploring the cost benefits of this technology ensuring all Australians can access state-of-the-art mechanical life support.
CRE ACTIONS has leveraged its research funding to over $9 million in conjunction with The University of Queensland, Metro North Hospital and Health Service, The Prince Charles Hospital Foundation, The Alfred, RPA, Monash University, The Queensland University of Technology, Griffith University, University of New South Wales and The Baird Institute for Applied Heart and Lung Surgical Research.
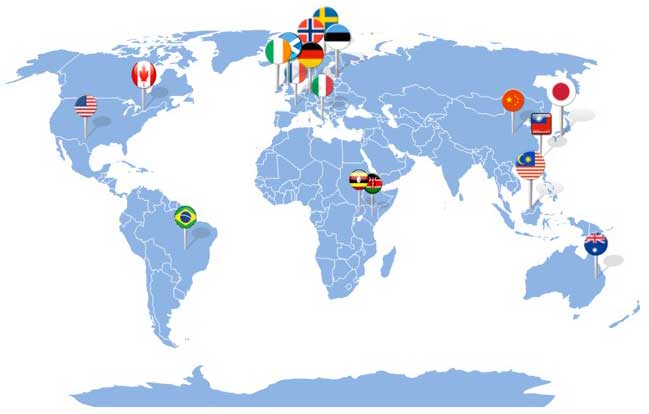
CRE ACTIONS has also evolved into a multi-national collaborative extending across Australia, USA, United Kingdom, Germany, France, Russia, Scandinavia, Malaysia, Singapore, China, Japan, Korea, Taiwan and Taipei which has now led to PhD scholarships from the world’s best mechanical support hospitals all coming to work at CRE ACTIONS – including Belfast, Toronto, The Alfred, and the Pitié-Salpetriere Hospital, Paris.
The assembled team of clinicians, nurses, scientists, engineers, allied health practitioners and research staff and students in the fields of critical care, paediatric intensive care, cardiothoracic and general surgery, cardiology, anesthesia, transplantation and thoracic medicine, internal medicine, emergency medicine, radiology, pathology, haematology, physiotherapy, speech therapy, perfusion, transfusion medicine, molecular and cellular science, echocardiography, pharmacological science and health care economics mirrors the large inter-disciplinary team that works with a patient and provides the perfect opportunity to work in a silo-free environment to achieve best research outcomes that lead to improved clinical outcomes for a critically ill patient.
CRE ACTIONS aims to ensure that not only do MADs achieve their full potential, but that the knowledge gained is put into practice in an optimal way, with appropriate training for patients and clinical staff. In addition there is a full understanding of health care costs which may be attributable to treating patients with these devices. Through this, CRE ACTIONS aims to advance the understanding in this field, and in doing so, improve the lives of patients – today and into the future.
Key Research Objectives:
- Understand the high impact pathological changes resulting from the body-device interface in patients supported by MADs.
- Improve the physiological interface of cardio-respiratory mechanical devices.
- Describe the changes in pharmacokinetics of drugs and nutrients essential to MAD therapy.
- Improve the patient experience of these physically and psychologically challenging interventions through consumer participation in research.
- Support development of public health policy with clinical practice guidelines and economic cost effectiveness analysis of MAD therapy.
The patient is the ultimate beneficiary of CRE ACTIONS research as innovations driven by clinical needs and cutting edge research into advanced organ support techniques with equal emphasis on qualitative aspects of intensive care patient management all lead to better clinical outcomes for a critically ill patient.
In 2015, the CRE ACTIONS successfully bid and won the right to host the third meeting of the Asia-Pacific Chapter of the Extracorporeal Life Support Organization (ELSO) in Brisbane in 2017.
ELSO was founded in 1989 and is an international consortium of health care professionals and scientists who are dedicated to the development and evaluation of novel therapies for support of failing organ systems. The Asia-Pacific Chapter endeavours to promote the safe practice and science of ECLS in the region, while engaging other centers worldwide in collaborative research.
AP ELSO 2017 – “ECMO and Beyond” will bring together an international consortium of health care professionals and scientists who are dedicated to the development and evaluation of novel therapies for support of failing organ systems and will showcase the work of CRE ACTIONS to the world.

Research
Cardiovascular disease is a leading cause of death, and is responsible for 17% of health expenditure in the USA and 11% in Australia (2008/9); the burden of disease it imposes in terms of disability and premature death is second only to cancer. It is responsible for more deaths (30% of all deaths) in Australia than any other disease group (Heart Foundation). The gold standard treatment of end-stage heart failure, heart transplantation, is limited by falling organ donation numbers.
The rapid and continuous expansion of the use of Mechanical Assist Devices (MADs), such as Extra Corporeal Membrane Oxygenation (ECMO) and Ventricular Assist Devices (VADs), in the management of acute and chronic end stage cardio-respiratory disease highlights the potential benefit to patients from research in this field. The barriers of complexity, cost and risk combine to inhibit the more widespread use of these devices.
Our research brings together expertise from around the world to investigate how to make mechanical hearts (MADs) more clinically viable.
By breaking down barriers across medical disciplines and geographic divides, CRE ACTIONS brings together researchers, clinicians educators and patients under a united cause: to conduct multi-disciplinary, collaborative research at the physiological and biological cardio-respiratory interface.
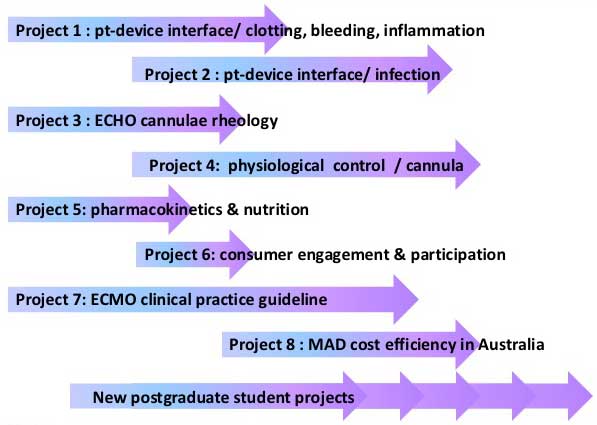
Our centre will undertake eight major research projects to make mechanical hearts a clinical reality.
Projects
Project 1
Scientific interrogation of the clinical complications of clotting, bleeding and blood transfusions associated with mechanical assist devices (MADs) through an in-vivo ovine model
Led by Professor Fraser, Project 1 will focus on the scientific interrogation of the clinical complications of clotting, bleeding and blood transfusion associated with MADS.
- Objective: Understand the high impact pathological changes in patients supported by MADs resulting from the body-device interface.
- Timeframe: 2015 – 2017
- Chief Investigator: CIA John Fraser
Project 2
Infections & biofilm formation after VAD implantation
Led by Professor McGiffin and Associate Professor Barnett, this project will study infections and biofilm formation after VAD implantation.
- Objective: Understand the high impact pathological changes in patients supported by MADs resulting from the body-device interface
- Timeframe: 2016 – 2019
- Chief Investigators: CIB David McGiffin and CIH Adrian Barnett
Project 3
Extend the use of echocardiography (ECHO) to improve visualisation of ventricular function, complications and cannula-cardiac interactions during mechanical assist device (MAD) therapy
Innovate ECHO Techniques:
- Project 3(a) Microsphere contrast ECHO
- Project 3(b) Intra-cardiac echocardiography (ICE)
- Project 3(c) 2D speckle tracking echocardiography (STE)
- Objective: Understand the high impact pathological changes in patients supported by MADs resulting from the body-device interface.
- Timeframe: 2015 – 2017
- Chief Investigators: CIA John Fraser, CIF Vincent Pellegrino, and CIE Geoff Tansley
Project 4
Improving the patient-device interface of cardio-respiratory mechanical assist devices (MADs)
(a) Physical patient MAD interface - sutureless canula
(b) Functional patient MAD interface
(c) Blood VAD interface
(d) Wearable components
- Objective: Improve the physiological interface of cardio-respiratory MADs therapy
- Timeframe: 2016 – 2019
- Chief Investigators: CIA John Fraser, CIC Nigel Lovell, CIE Geoff Tansley and CII: Shaun Gregory
- Associate Investigators: AIB Toru Masuzawa, AIC Suhaini Kadiman, AID David Platts, AIE Kiran Shekar, AIF Einly Lim, AII Daniel Timms and AIJ Christopher Hayward
Project 5
(a) pharmacokinetic analysis of key drugs in patients using ex-vivo circuits and an in-vivo animal model; and (b) investigation of nutrients and trace element levels in ECMO patients
- Objective: Explicitly describe the changes in pharmacokinetics of drugs and nutrients essential to MAD therapy
- Timeframe: 2015 – 2017
- Chief Investigator: CIG Jason Roberts
- Associate Investigators: AIE Kiran Shekar and AIH Maree Smith
Project 5(a)
Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation (ASAP ECMO): An international multi-centre study to optimise drug dosing and improve patient outcomes
The Right dose of the Right drug, at the Right time
Endorsed by the international ECMO Network (ECMOnet)
Chief Investigator: A/Prof Kiran Shekar
Extracorporeal membrane oxygenation (ECMO) is among the most rapidly increasing life support techniques used in intensive care. Its use in the United States has increased more than 433% in a space of only 5 years6. ECMO is highly versatile and not only provides temporary cardiorespiratory support in the acute setting, but it also effectively complements cardiac surgical and interventional cardiology procedures, timely implantation of long-term cardiac assist devices, heart and lung transplantation programmes and cardiopulmonary resuscitation4,7. ECMO is considered a supportive therapy, whilst drug therapy is used to treat the underlying disease and minimise complications 8. However, ECMO is thought to affect the pharmacokinetics (PK) of many drugs making it difficult to provide optimal drug therapy to reverse the underlying disease8. For many important drugs, our research thus far illustrates an interaction between the drug and the artificial surfaces of the ECMO device9-16. This results in significantly altered drug concentrations in the most severely ill patients who already have profound PK changes. Given the morbidity and mortality associated with suboptimal antibiotic and sedative therapy in ICU patients, altered PK of these drugs 9-13 is of high clinical relevance.
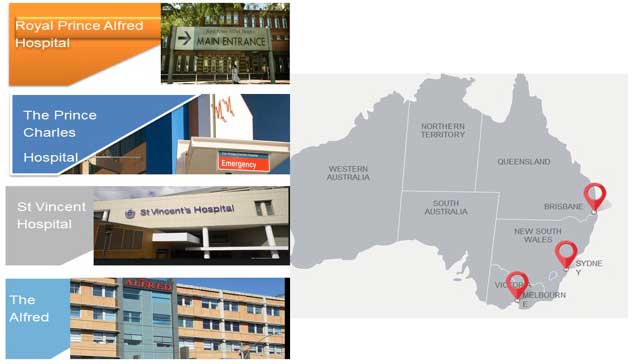
This international, multi-centre population PK study aims to develop evidence based dosing guidelines for 18 important antibiotic, sedative and analgesic drugs during ECMO. Patients receiving ECMO at the Prince Charles Hospital, Brisbane; St Vincent’s Hospital, Sydney; The Alfred, Melbourne; Auckland City Hospital, Auckland; New York Presbyterian Hospital, New York and Erasme Hospital, Belgium will be enrolled. This study will provide critical PK data to guide clinicians in administering “the right dose of the right drug, at the right time” during ECMO. Given that the investigators are ECMO and PK experts who play vital roles within the extracorporeal life support organisation (ELSO), the international ECMO Network (ECMONet) and the NHMRC Centre for Research Excellence in Advanced Cardio-respiratory Therapies Improving Organ Support (ACTIONS 2015-9), data from these studies will be translated into clinical practice rapidly, facilitating optimal drug therapy, minimising the risk of therapeutic failure and the rise of resistant pathogens due to suboptimal antibiotic concentrations. We will generate new knowledge to allow a more refined application of ECMO by providing the first robust dosing guidelines for these complex patients.
Project 6
Develop informed, trained consumer groups whose members participate as partners in our CRE research projects and processes
- Objective: Improve the patient experience of physically & psychiologically challenging MAD interventions through consumer participation in CRE research
- Timeframe: 2016 – 2019
- Chief Investigators: CIA Fraser (ECMO) and CIB McGiffin (VAD)
Project 7
Develop an NHMRC clinical practice guideline on the use of MAD therapy for the Australian healthcare setting, which incorporates evidence and new knowledge generated by the ACTIONS CRE
- Objective: Support development of public health policy with MAD NHMRC clinical practice guidelines & economic cost effectiveness analysis of MAD therapy
- Timeframe: 2015 – 2019
- Chief Investigators: CID Paul Bannon and CIA John Fraser with input from CIF Vincent Pellegrino
Project 8
Determining the economic cost effectiveness of MAD therapies in Australia
- Objective: Support development of public health policy with MAD NHMRC clinical practice guidelines & economic cost-effectiveness analysis of MAD therapy
- Timeframe: 2017 – 2019
- Chief Investigator: CIH Adrian Barnett with input from CID Paul Bannon, CIG Jason Roberts and CIF Vincent Pellegrino
Projects framework
Our performance
- 100+ publications
- 76+ presentations
- 34+ research students
Clinical centres at CRE nodes
Contact
The Clinical Sciences Building
The Prince Charles Hospital
627 Rode Road
Chermside Queensland 4032
Australia
Office Hours
8:30am - 4:30pm, Monday to Friday
General enquiries
Phone: +61 (7) 3139 6880